Introduction
Pre-transplant leukemic load significantly impacts relapse and long-term survival in patients with acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS). A prolonged duration of busulfan administration was reported as a safe myeloablative option with less toxicity and allows the addition of epigenetic agents, like chidamide, which may be synergistic with conditioning chemotherapy and may further improve disease control. Meanwhile, Pre-conditioning busulfan may play a priming role in enhancing the sensitivity of tumor cells to the following conditioning therapy. Here we report the safety and preliminary efficacy of chidamide and fractionated busulfan regimen in patients with AML or MDS who could not clear leukemia cells before transplantation.
Methods
We enrolled AML or MDS patients with residual or active disease after at least one line of standard therapy. Eligible patients received fractionated busulfan at a total dose of 12.8 mg/kg within 2 weeks. The first two doses of busulfan (1.4 mg/kg IV each) were administered on days -14 and -13. The rest of the busulfan was given on days -7 to -4. Fludarabine and cytarabine were given on days -7 to -3. Chidamide 30 mg twice weekly was given from day -14 to + 3. Peripheral stem-cell were used as grafts harvested from HLA-matched sibling donors (MSD), matched unrelated donors (MUD), or haploidentical donors (HID). Post-transplantation acute graft versus host disease (GVHD) prophylaxis consisted of cyclophosphamide on day +3 to +4, and cyclosporine started on day +5. For HID transplant recipients, mycophenolate mofetil was added from day +5 to day +35. Follow-up examinations were scheduled at +1, +2, +3, +4, +6, +9, +12, +18, and +24 months post-transplant. Donor lymphocyte infusion was not administered prophylactically in this trial.
Results
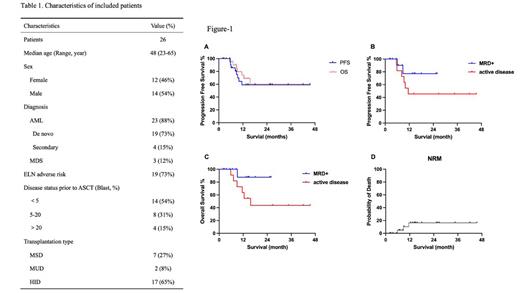
Twenty-six patients with de novo AML (n=19), secondary AML transformed from MDS (n=4), or MDS-EB2 (n=3) were enrolled with a median age of 48 years (range, 23~65 years). Patient and disease characteristics are summarized in Table 1. Nineteen patients had adverse risk karyotype according to European LeukemiaNet risk stratification. They received MSD (n=7), MUD (n=2), or HID (n=17) HSCT. Pre-transplant tests revealed that 14 of the patients had MRD (n=14, blast cells<5% in the bone marrow), and 12 had active disease (blast cells ≥5%, including 4 who had blast cells ≥20%). The median pre-transplant blast cells were 7.5%. All patients successfully finished scheduled conditioning. Neutrophils and platelets engrafted at a median of 13 days (range,11-18 days) and 16 days (range, 11-53 days) post-transplant, respectively. All patients reached MRD negative with full donor chimerism at day +30. Cumulated incidence of grade II to IV and grade III to IV acute GvHD were 12.5% and 5.3% respectively. The median follow-up time was 13 months (2-45.5 months). For the whole cohort, the estimated 1-year progression free survival (PFS) and overall survival (OS) were 59.1% and 69.0% (Figure-1A). For patients with MRD+ or active disease, estimated 1-year PFS are 77.1% and 45.5%, respectively (Figure-1B), and estimated 1year OS of these two groups of patients are 87.5% and 63.6%, respectively (Figure-1C). Three patients died from pulmonary infections (2 with COVID-19 and 1 with cytomegalovirus) in CR at 5.8, 6.9, and 11.5 months post-transplant. The incidence of non-relapse mortality at 6 months and 1 year post-transplant was 4.8% and 16.3% respectively (Figure-1D).
Conclusion
Fractionated-busulfan based myeloablative conditioning regimen with chidamide confers survival advantage and good tolerance in acute leukemia and MDS patients with residual or active disease.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal